Since conversion in the reactor is only 15 a recycle stream is used and in order to avoid build-up of inerts a purge stream is withdrawn. 3 at 50 atm and 6 5 0 is allowed to react.
How Many Grams Of Nh3 Are Produced By 4 Moles Of N2 And 10 Moles Of H2 Quora
Answer 1 of 2.
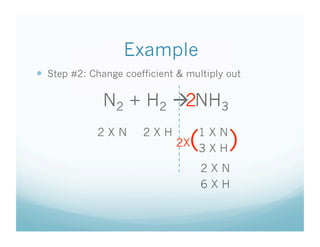
. 18x 32 x 916. 3 mol H_2. 1 Balance this equation.
Which has the highest root-mean-square speed vrms for their molecules. From the equation we can see that 1 mole of N2g reacts with 3 moles of H2g or 3 moles of H2g react with 1 mole of N2g to produce 2 moles of NH3g. H2 H2S H2 S H2S S.
Calculate the ΔG of mixing when you remove the barrier. N2 H2 N2 NH3 H2 NH3. 2 Balance this equation.
What is the mole ratio for NH3. For this reaction the balanced chemical equation is. N2 H2 N2 NH3 H2 NH3.
N2 H2 --- NH3 write the following molar ratios. Part A Using the following chemical reaction determine the mole ratio needed to convert from moles of N2 to moles of NH3. N2 9H2 g NH3 g View Available Hint s O2 mole Na1 mole NHs 1 mole N1 mole NH O1 mole N2 mole NH.
3 Write and balance the equation for the synthesis of water. N2g 3H2g ---- 2NH3g 1mol3mol. Then answer the following questions.
The molar masses of H2 and N2 are 20 gmol and 280gmol respectively. H2 S --- H2S write the following molar ratios. 1 Balance this equation.
Calculate the equilibrium constant Kp of the. 1 mol O_2. 1 mol n_2.
If the equilibrium pressure is p the partial pressure of NH3 at equilibrium is. 2 Balance this equation. 1 Balance this equation.
N 2 g 3H 2 g 2NH 3 g for the reaction initially the mole ratio was 13 of N 2 H 2. H2 S --- H2S write the following molar ratios. The mole ratio can be determined by first writing out a balanced chemical equation for the reaction.
Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. At the start of the reaction the Pressure wasP. Two gases H2 and N2 are the same temperature.
Then answer the following questions. N2 H2 N2 NH3 H2 NH3. H2 S --- H2S write the following molar ratios.
Why do we know that in the balanced chemical equation C O2 --- CO2 1 g of C will not react exactly with 1 g of O2. How many moles of P4 are needed to react with 9 moles of Mg. 3 mol H_2 1 mol N_2 3 mol H_2 or 3 mol H_2 1 mol N_2 1 mol N_2.
How many moles of O₂ are required to form 500 moles of H₂O. Thus the mole fraction of N2 316 H2 916 and NH3 14 respectively. 2 mol H_2O The mole ratios for each substance pair would be 2 mol H_2.
The product stream consists of pure ammonia. Mole Ratio Worksheet - Oswego Community. The pressure on each side of the box is 1 atm.
Science Chemistry QA Library The Haber process involves the following reaction. B Now move the barrier in the box from part a. For this reaction the balanced chemical equation is 2H_2 O_2 - 2H_2O The mole ratios are determined using the coefficients of the substances in the balanced chemical equation.
N₂ 3H₂ 2NH₃. Reaction n23h2 2nh3 the ratio by volume of n2 h2 and nh3 is 132 this illustrates the law of Gay Lissas law. Chemistry questions and answers.
N2 H2 --- NH3 write the following molar ratios. 1 mol O_2 2 mol H_2 1 mol O_2 or 1 mol O_2 2 mol H_2 2 mol H_2. N2 3 h2 2 nh3 write the following molar ratios.
Molar mass of N2H2 is 3002928. From the stoichiometry of the reaction 3 moles of hydrogen is required to react with 1 moles of Nitrogen to give two moles of Ammonia NH3. The mole fraction He is 04 in gaseous mixture He and CH4.
Using the following chemical reaction determine the mole ratio needed to convert from moles of N2 to moles of NH3. N2g H2g NH3g 1 mole N22 mole NH3. H2 H2S H2 S H2S S.
The equilibrium mixture was found to contain 15 of ammonia and the total pressure in the container is 10 atm. N2 H2 --- NH3 write the following molar ratios. 3 Write and balance the equation for the synthesis of water.
H2 H2S H2 S H2S S. At equilibrium 50 of each has reacted. Suppose if we consider 1 mole of N2 gas and 3 moles of H2 gas was present in the beginning4 moles After the completion of 50 of the Reaction.
The rate of purge stream is adjusted to keep inert concentration in the recycle stream at 8 mole per cent. The molar ratio of H2N2 is 31. N2 3 H2 --- 2 NH3 write the following molar ratios.
2 Balance this equation. 08 x 32 x 14. 2 mol H_2.
2 mol NH_3 The mole ratios for each substance pair would be 1 mol N_2. The Moles are the coefficients of the molecules in the reaction equation. Click hereto get an answer to your question N2 g 3H2 g 2NH3 g for the reaction initially the mole ratio was 13 of N2H2.
For this reaction the balanced chemical equation is N_2 3H_2 - 2NH_3 The mole ratios are determined using the coefficients of the substances in the balanced chemical equation. Calculate any molar masses formula weights needed and fill in the numbers using the balanced equation to find the molar ratios. 06 06 x 18 x 08x 06 32 x 3 16.
3 rows A mixture of N 2 and H 2 is the molar ratio 1. Assume all gases are initially at standard temperature and pressure and that they can be treated as ideal. Then answer the following questions.
A mixture of N2 and H2 are in the molar ratio 13 is allowed to attain equilibrium when 50 of mixture has reacted If rm P is the equilibrium pressure then partial pressure of NH3 formed is A dfracP6 B dfracP2. At equilibrium 50 of each has reacted. Two gases H2 and N2 are the same temperature.
A We have one mole each of H2 and N2 separated by an impermeable barrier in a box. N2 g 3H2 g 2NH3 g N2 and H2 in the mole ratio of 13 were mixed in a closed container and allowed to achieve equilibrium at 450 oC. 6 Mg P4 2 Mg3P2.
A N2 H2 b N2 NH3 and c H2 NH3. 3 Write and balance the equation for the synthesis of water. The moles ratio of Hydrogen to Ammonia is 32.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O.

Solved Mole Ratio Worksheet 1 Given This Cquation N2 3h2 Chegg Com
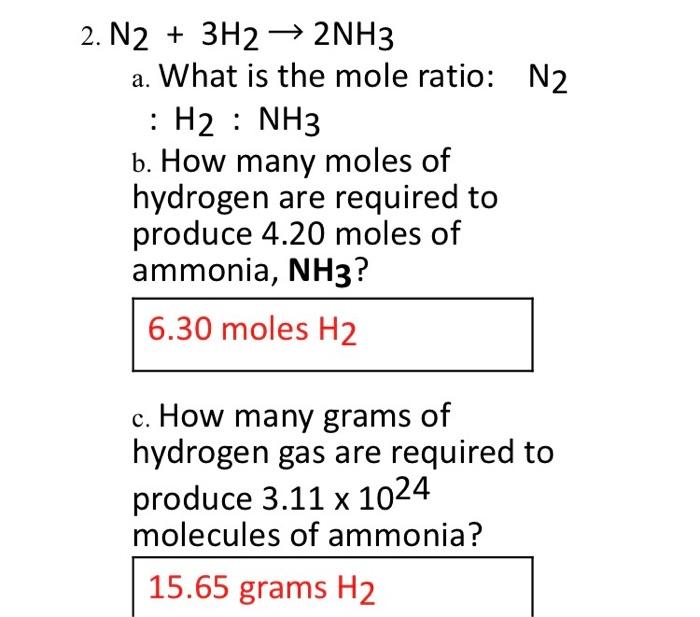
Solved 2 N2 3h2 2nh3 A What Is The Mole Ratio N2 H2 Chegg Com
0 Comments